










About us
About Sadguru Technical Solution
Sadguru Technical Solution is a professional service provider offering end-to-end technical documentation solutions for manufacturers in the herbal, nutraceutical, Ayurvedic, cosmetics and food industries. The company plays a critical role in supporting businesses to ensure their products meet regulatory compliance, both for domestic and international markets.
With offices strategically located in Pali, Sojat, and Jodhpur, Sadguru Technical Solution is well-positioned to serve manufacturing hubs across Rajasthan and neighboring states. These regions are known for their strong presence in Ayurvedic and herbal product manufacturing, and Sadguru Technical Solution acts as a vital support system for companies operating in these sectors.
Core Document Services
For a GMP (Good Manufacturing Practices) and factory setup, comprehensive documentation is essential to demonstrate compliance with regulatory requirements and quality standards. Below is a categorized list of documents typically required, based on Domestic and international GMP guidelines:
Manufacturing Documents
Master Manufacturing Formula (MMF) / Batch Manufacturing Records (BMR)
Batch Packaging Records (BPR)
Raw Material and Finished Product specifications, Method of analysis and Certificate of analysis
Packing materials specifications, Method of analysis and Certificate of analysis
Process Validation Protocols & Reports
Stability studies and Product development Report
Equipment Qualification (DQ, IQ, OQ, PQ)
Calibration records
Facility and Equipment Documents
Site Master File
Layout plan of facility (with material and personnel flow)
HVAC qualification and monitoring reports
Water system validation reports
Preventive Maintenance records
Cleaning and Sanitation SOPs & logs
Pest control SOPs & records
Quality Management System (QMS) Documents
Quality Manual
Quality Policy & Objectives
SOPs (Standard Operating Procedures)
Product Quality Review (PQR)/Annual Product Review (APR)
Quality Risk Management reports
Deviation Reports and CAPA (Corrective and Preventive Actions)
Change Control records
Material Management Documents
Vendor Qualification documents
Material Receipt, Testing, and Release records
Material Inventory logs
Warehousing SOPs
Quarantine and Rejection procedures
Documentation Control
SOP for Document Control
Master List of SOPs
Document revision history
Controlled document issuance and retrieval logs
Regulatory and Product Registration Documents
Dossiers (CTD/ACTD format if applicable)
Product Labels & Artwork approvals
Regulatory correspondence records
Environmental Monitoring & Utilities
Environmental Monitoring SOPs & Data
Water, air, and surface sampling records
HVAC qualification reports
Utility Validation
Safety & Emergency Preparedness
MSDS (Material Safety Data Sheets)
Incident/Accident Reports
Fire & Safety drill records
Emergency SOPs
Personnel Documents
Organizational Chart
Job Descriptions
Training records (Initial & Ongoing GMP Training)
Health and Hygiene SOPs
Gowning SOPs
Our Story
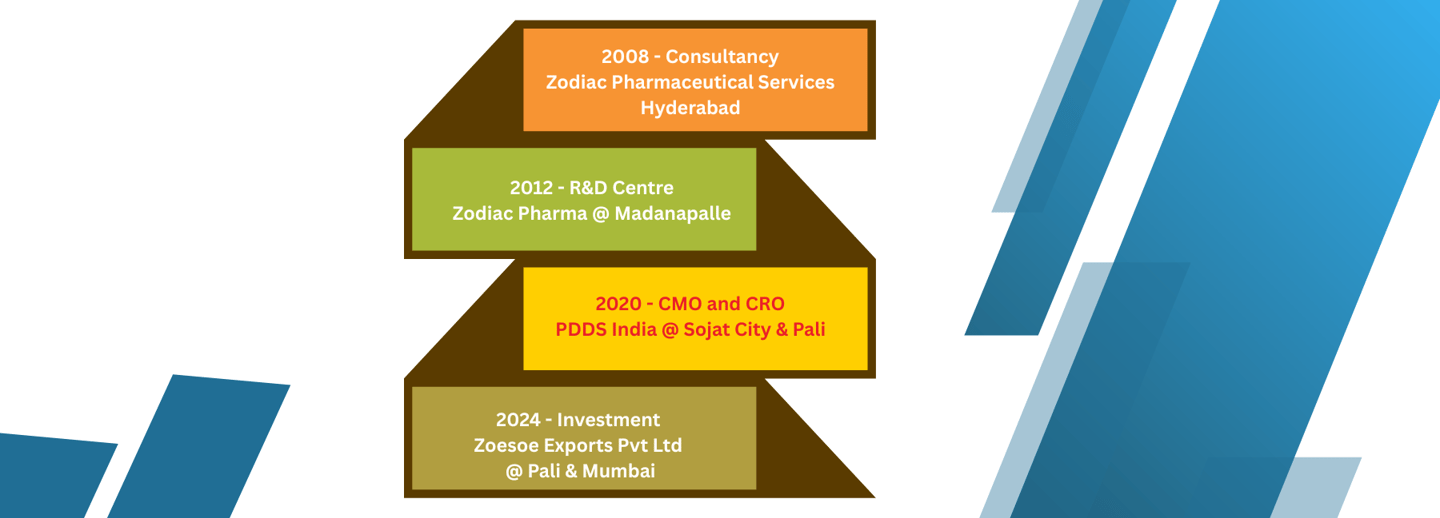
🔹 2008 – Inception as Consultancy
Zodiac Pharmaceutical Services, Hyderabad
Started as a pharmaceutical consultancy service offering technical and regulatory solutions.
🔹 2012 – Expansion into Research & Development
Zodiac Pharma R&D Centre, Madanapalle
Established a dedicated R&D facility to support formulation development, analytical method development, and innovation.
🔹 2020 – Vertical Integration: Manufacturing & Clinical Services
PDDS India, Sojat City & Pali
Initiated operations as a Contract Manufacturing Organization (CMO) and Contract Research Organization (CRO), offering regulatory, dossier, and technical writing services.
🔹 2024 – Strategic Investment in Global Exports
Zoesoe Exports Pvt Ltd, Pali & Mumbai
Expanded into global markets with investment in herbal, food, and cosmetic product exports under the brand Zoesoe Naturals.
150+
18+
Years of experience
Happy clients

Our Founder’s Journey
Mr. T. C. Tak, a visionary in the pharmaceutical and natural products industry, began his journey with a clear mission — to bridge the gap between regulatory compliance, research innovation, and global market access for Indian manufacturers. Mr. T. C. Tak's entrepreneurial journey reflects his unwavering commitment to quality, compliance, and innovation. From regulatory consultancy to research, manufacturing, and global exports, his ventures have empowered numerous Indian manufacturers to meet international standards and achieve global success.
Linkedin Profile
http://linkedin.com/in/tctak +15000 connections

Services
Expert support for compliance and product quality.
Zoesoe Exports Pvt Ltd - Our Investment partner
Support / Connect
enquiry
+91-8440009911 +91-96522-81814
© 2025. All rights reserved.
Plot No 40, Kharsa No 2051/33 , PDDS Building, Near Sojat Road Marg, Landmark, NH162, Sojat, Rajasthan 306104
Plot No. G6 , RIICO Industrial Area, Naya Gaon, NH162 District- Pali, Rajasthan, 306401 , India
